Regeneron Monoclonal Antibody Treatment
Cost of the Regeneron treatment is covered by the federal government according to a company spokesperson. About Regeneron Antibody Cocktail Casirivimab and imdevimab injection is a cocktail of two monoclonal antibodies also known as REGN10933 and REGN10987 respectively and was designed specifically to block infectivity of SARS-CoV-2 the virus that causes COVID-19.

Monoclonal Antibodies Overburdened Hospitals Are Not Offering This Treatment Against Covid 19 The Washington Post
Regeneron is collaborating with Roche to increase global supply of REGEN-COV with expected production of at least 2 million treatment doses per year beginning in 2021.
Regeneron monoclonal antibody treatment. They can be administered through IV or injection. What are Monoclonal Antibodies. REGN today welcomed positive results from the largest trial assessing any monoclonal antibody treatment in patients hospitalized with severe COVID-19.
The medicine is called Regeneron or REGEN-COV which consists of two monoclonal antibodies. Food and Drug Administration recently expanded the use of Regenerons monoclonal antibody COVID-19 treatment to include prevention. Monoclonal antibodies are laboratory-made proteins that duplicate the immune systems ability to fight off viruses.
The two treatments by Eli Lilly and Regeneron are the first drugs developed specifically for Covid-19 to be authorized by the FDA. November 23 2020 -- The FDA issued an emergency use authorization to Regeneron Pharmaceuticals for its monoclonal antibodies -- casirivimab and imdevimab -- to be given together to treat. Monoclonal antibody treatments can help prevent severe disease.
The Randomised Evaluation of COVID-19 Therapy RECOVERY trial has demonstrated that the investigational antibody combination developed by Regeneron reduces the risk of death when given to patients hospitalised with severe COVID-19 who have not mounted a natural antibody response of their own. They consist of artificially synthesized copies of the. Outpatient treatment is now available for patients with mild-to-moderate COVID-19 symptoms.
It contains the monoclonal antibodies casirivimab and. Read on to learn about this kind of treatment how it works when its most useful and who can benefit. Updates to the ACIP recommendation may be made as additional information on the interaction between prior monoclonal antibody treatment.
Casirivimabindevimab from Regeneron is a monoclonal antibody combination that is infused directly into a vein. On Friday November 21 the Food and Drug Administration FDA authorized the use of Regenerons monoclonal antibody cocktail for the treatment of COVID-19. The treatment uses a combination of two monoclonal.
After treatment with a monoclonal antibody for COVID-19. EUA be expanded to include appropriate hospitalized patients. The first dose of Regenerons cocktail must be injected within 96 hours of exposure to the coronavirus the FDA said in a statement.
Regeneron is responsible for development and distribution of the treatment in the US and Roche is primarily responsible for development and distribution outside the US. Health care providers should review the Antiviral Resistance information in Section 15 of this Fact Sheet for details regarding specific variants and resistance and refer to the CDC website. A monoclonal antibody treatment developed by the drug maker Regeneron sharply cut the risk of hospitalization and death when given to high-risk Covid-19 patients in a large clinical trial the.
What is monoclonal antibody treatment. Circulating SARS-CoV-2 viral variants may be associated with resistance to monoclonal antibodies. Monoclonal antibodies are proteins that mimic the immune systems ability to fight COVID-19.
The results could pave the way for Regenerons monoclonal antibody treatment to be approved by UK regulators and rolled out for use across the. Regeneron will share new data with regulatory authorities immediately and request that the US. The drug referred to as REGN-COV2 is the same one that President Trump received after being diagnosed with COVID-19 in early October.
The treatment uses a cocktail of two monoclonal antibodies casirivimab and imdevimab known as Regen-Cov in the US that bind specifically to two different sites on the coronavirus spike.

Fda Authorizes Regeneron S Monoclonal Antibody Treatment For Covid 19 Rt A Medqor Brand
News Recovery Trial Monoclonal Antibody Treatment Saves Lives Of Seronegative Hospitalised Covid 19 Patients Nihr
Devexplains Monoclonal Antibody Treatment For Covid 19 Devex

Experimental Covid Treatment Expands In Lehigh Valley The Morning Call

Fda Approves Emergency Use Of Monoclonal Antibody For Treatment Of Covid 19
Monoclonal Antibodies Could Ease Record Covid Hospitalizations Why Are They Going Unused

Monoclonal Antibody Treatment Idph
Covid 19 Treatment Options Banner Health

What Are Monoclonal Antibodies And Can They Treat Covid 19 Iav
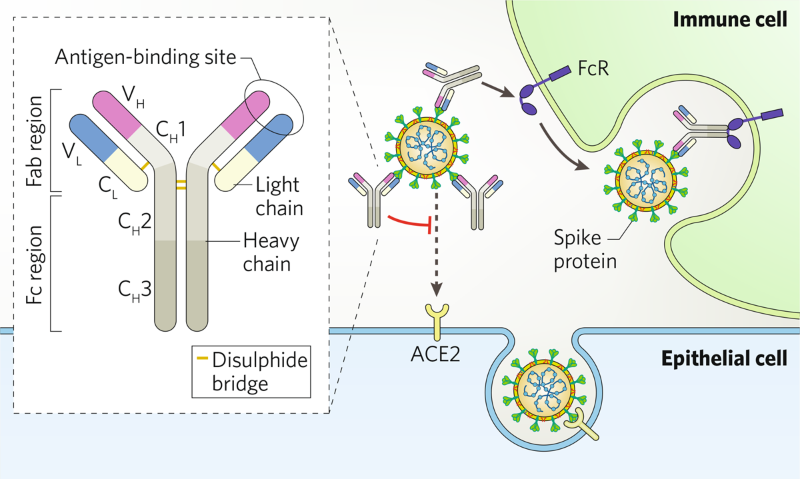
Hunting For Antibodies To Combat Covid 19
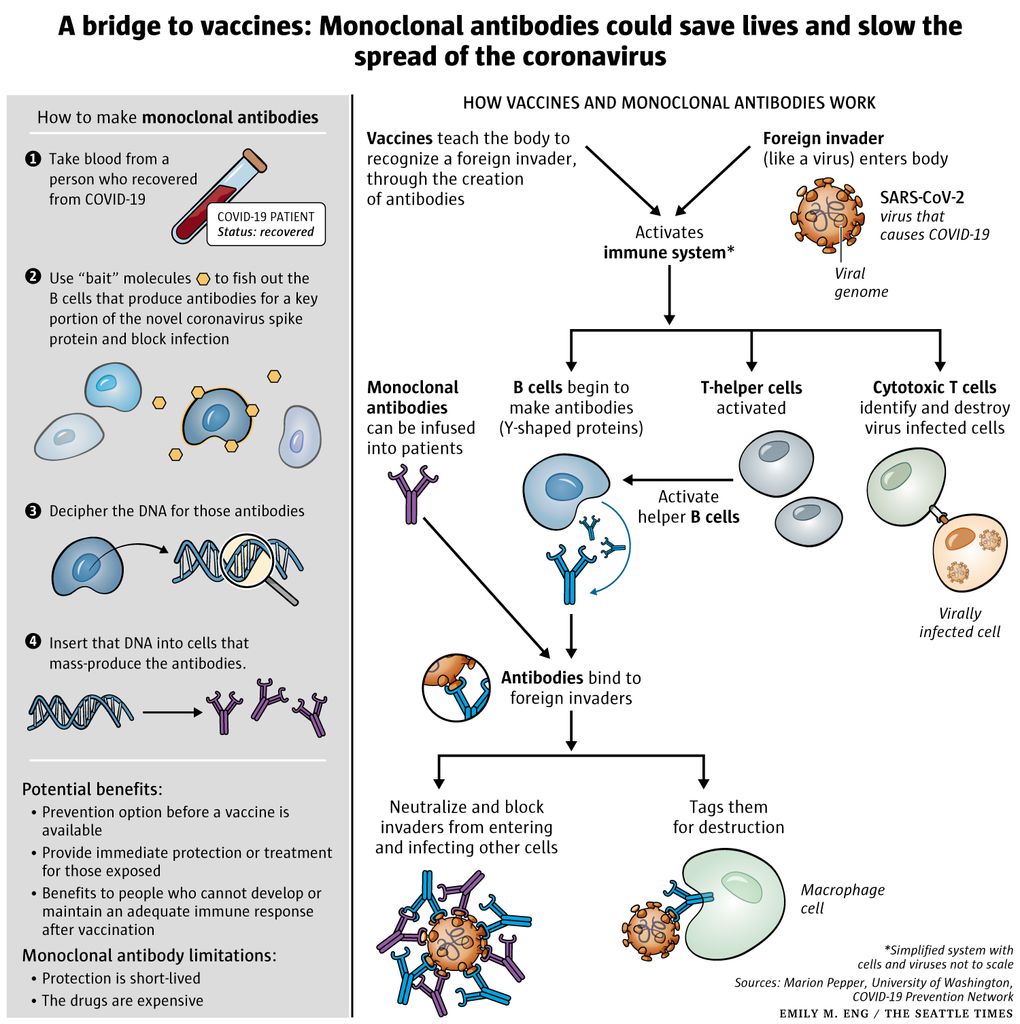
Monoclonal Antibodies Could Fill The Covid 19 Treatment Gap Until Vaccines Arrive But At A Cost The Seattle Times

Vaccine Delays In Europe Lead To German Direct Purchase Of 486m Of Lilly Regeneron

Covid In Florida Gov Desantis Launches Monoclonal Antibody Treatment Centers Cbs Miami





Post a Comment for "Regeneron Monoclonal Antibody Treatment"